Introduction:
Our teeth are exposed to numerous environmental influences every day. Acids from food and drinks attack our tooth enamel in the same way as bacteria do. It is therefore important that our teeth are well protected. Several studies have already shown that after using products with hydroxyapatite, these particles accumulate on the tooth surface: A protective layer is created. So far, however, it has not yet been clarified what percentage of the tooth surface is covered with hydroxyapatite products after a single application.
Question
What is the degree of coverage of the tooth surface with hydroxyapatite after a single application?
material and methods
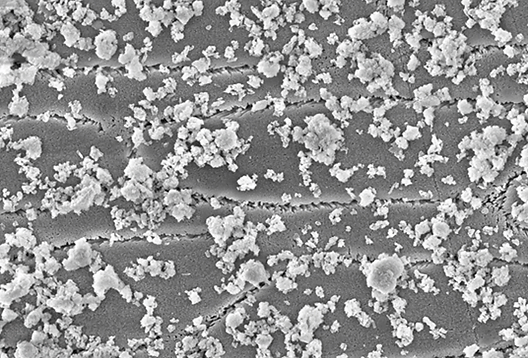
This in vitro study was carried out at the Max Planck Institute. Tooth enamel flakes from bovine teeth were immersed in aqueous solutions containing hydroxyapatite particles. The following concentrations of hydroxyapatite particles were used: 1, 5 and 10%. The platelets were then examined using a scanning electron microscope. The degree of coverage of the tooth surface with hydroxyapatite was measured and quantified.
Results
The tooth samples were covered with microcrystalline hydroxylapatite particles after just one immersion in an aqueous solution. With a 10% hydroxyapatite dispersion, more than a third of the surface was covered with a layer of hydroxyapatite after a short time. The microscopic images also indicate that the particles are firmly anchored on the tooth surface.
conclusion
After a single use of dental care products with hydroxyapatite, a protective layer forms on the tooth surface. This layer makes it difficult for bacteria and acids to attack the natural tooth enamel.